When you're living with Crohn's disease or ulcerative colitis, finding the right treatment isn't just about reducing symptoms-it's about reclaiming your life. For many people, conventional drugs like steroids or immunomodulators don't cut it. That's where IBD biologics come in. These aren't your typical pills. They're precision-targeted medicines designed to quiet the overactive immune response that attacks your gut. And today, there are more choices than ever: anti-TNF agents, anti-integrins, and IL-12/23 inhibitors. Each works differently. Each has trade-offs. And choosing the right one can make all the difference.
What Are IBD Biologics?
IBD biologics are made from living cells, not chemicals. They mimic proteins your body naturally makes to calm inflammation. Instead of broadly suppressing your immune system like older drugs, they zero in on specific troublemakers-like TNF-alpha, integrins, or interleukins-that drive the damage in your intestines. The first one, infliximab (Remicade), hit the market in 1998. Since then, dozens of studies and real-world data have shown these drugs can bring deep remission, heal gut lining, and cut hospital visits in half. But they’re not magic. They’re powerful. And they come with real risks and logistics you need to understand.
Anti-TNF Inhibitors: The OGs of IBD Treatment
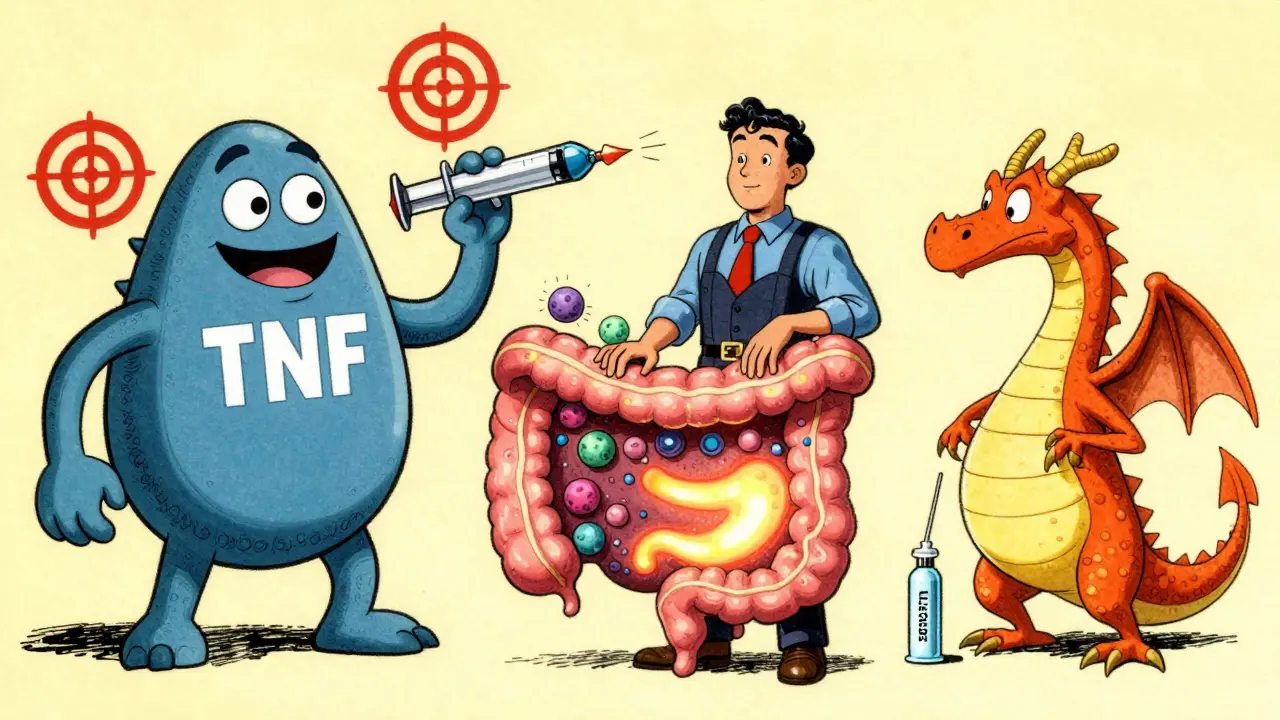
Anti-TNF drugs were the first to show that targeting one immune molecule could transform outcomes. They include infliximab (Remicade), adalimumab (Humira), golimumab (Simponi), and certolizumab pegol (Cimzia). These are still the most widely used biologics, making up about 65% of the global IBD market. Why? Because they work fast. Most people start feeling better in 2 to 4 weeks. In clinical trials, infliximab has consistently shown higher rates of remission and mucosal healing than other biologics in patients who’ve never tried one before.
But there’s a catch. These drugs circulate throughout your body. That means while they’re calming your gut, they’re also lowering your defenses everywhere. The FDA warns of increased risks for serious infections-like tuberculosis or fungal infections-and rare but dangerous conditions like lymphoma. Infusion reactions are common with infliximab: about 42% of users report rashes, itching, or chills during or right after the infusion. Adalimumab, the self-injectable version, avoids the clinic but causes injection site pain in up to 30% of users. And if your body starts making antibodies against the drug? That’s loss of response. About 6-25% of patients eventually lose effectiveness, often requiring dose changes or switching.
Anti-Integrin Therapy: Gut-Selective and Safer
Enter vedolizumab (Entyvio). This drug doesn’t touch your whole immune system. It blocks a specific molecule-α4β7 integrin-that only lets immune cells enter the gut. Think of it like a bouncer at a club: it says no to the bad cells heading to your intestines, but lets them roam freely elsewhere. That’s why it’s one of the safest biologics out there. No increased risk of brain infections like PML (unlike natalizumab, which was pulled for this reason). No higher risk of lung or liver infections. And fewer systemic side effects overall.
But it comes with a trade-off: slower action. It takes 6 to 10 weeks to see full effect. That’s hard for someone in pain. Still, in real-world data from MyIBDTeam, 72% of users reported effectiveness, and only 18% had side effects-far lower than anti-TNFs. A 2022 meta-analysis found vedolizumab was nearly as good as infliximab for inducing remission, especially in patients who didn’t respond to anti-TNFs. It’s also preferred for people with psoriasis (because anti-TNFs can make it worse) or those with a history of latent TB. The catch? You need an IV every 8 weeks. That’s 3-5 hours at a clinic. For some, it’s worth it. For others, it’s a dealbreaker.
IL-12/23 and IL-23 Inhibitors: The New Frontier
The newest class-IL-12/23 and IL-23 inhibitors-takes a smarter approach. Ustekinumab (Stelara) blocks both IL-12 and IL-23. Risankizumab (Skyrizi) and mirikizumab (Omvoh) target only IL-23, which is now seen as the key driver in IBD inflammation. These drugs are subcutaneous injections, given every 8 or 12 weeks. No infusions. No weekly shots. Just a simple injection, often at home.
The data is strong. Risankizumab, approved for ulcerative colitis in June 2024, showed 29% of patients achieved clinical remission at 52 weeks-nearly triple the placebo rate. Mirikizumab, approved for UC in 2022, had similar results. And safety? Cleaner than anti-TNFs. No black box warnings. No increased lymphoma risk in trials. In fact, the FDA doesn’t require special monitoring for these drugs like it does for TNF inhibitors.
But they’re expensive. A single 300mg dose of vedolizumab costs about $5,500. A 130mg dose of ustekinumab? Around $7,200. Insurance helps, but out-of-pocket costs still hit 41% of patients hard. That’s why manufacturer programs like Janssen CarePath or AbbVie’s patient support are critical. Many pay $0 to $5 per dose with help.
Which Biologic Is Right for You?
There’s no one-size-fits-all. But here’s how experts weigh in:
- If you need fast, powerful results-and you’re young, with moderate-to-severe disease-infliximab still has the strongest evidence. It’s the go-to for many gastroenterologists.
- If convenience matters more-you work full-time, have kids, or hate clinics-adalimumab or ustekinumab might be better. Self-injections beat weekly trips to the infusion center.
- If you’ve tried anti-TNFs and failed-vedolizumab or ustekinumab are top next steps. Studies show over 50% respond even after TNF failure.
- If you have other autoimmune conditions-like psoriasis or MS-vedolizumab or IL-23 inhibitors are safer. Anti-TNFs can flare psoriasis. Natalizumab (not used for IBD anymore) caused brain damage. IL-23 drugs? Clean slate.
Dr. Adam Cheifetz, a leading IBD specialist, says infliximab remains the first-line choice for bio-naive Crohn’s patients. But Dr. Laurie Keefer reminds us: "Convenience can outweigh marginal efficacy differences." For many, that means choosing a drug that fits your life-not just your lab results.
Real Talk: What Patients Say
On MyIBDTeam, infliximab users praise its power but complain about the 8-hour round trip to the clinic. One wrote: "It saved my colon, but I lost my weekends." Adalimumab users love the freedom but dread the red, swollen injection sites. Vedolizumab gets high marks: "Took 10 weeks to work, but once it did? I could eat again. No more hospital stays."
A Reddit user named CrohnsWarrior87 switched from Humira to Entyvio after five years: "No more weekly injections. But waiting 10 weeks? Brutal. I thought I’d never feel better." Another, UC_Survivor2023, said: "Remicade worked in two weeks. But the travel? Unbearable long-term."
Cost is a silent crisis. Even with insurance, 41% of patients say they still struggle to afford biologics. Many rely on patient assistance programs. If you’re eligible, apply. These programs can cut costs by 90%.

What You Need to Know Before Starting
- Vaccines first: Get all age-appropriate shots-flu, pneumonia, shingles, HPV-before starting any biologic. Live vaccines (like MMR or varicella) are off-limits once you’re on treatment.
- Screen for TB: All patients get a skin test or blood test before starting anti-TNFs. Latent TB can flare into active disease.
- Watch for infections: Fever, chills, cough, or unexplained fatigue? Call your doctor. Don’t wait.
- Injection training: If you’re on adalimumab or ustekinumab, your nurse will walk you through it. Most people master it in one or two tries. But 22% develop anxiety. Ask for support.
- Track your symptoms: Use apps like MyTherapy. 68% of users say they stick to their schedule better with reminders.
The Future: What’s Next?
The market is exploding. IL-23 inhibitors are growing at 25% a year. By 2028, they could make up 30% of the IBD biologic market. New drugs like etrolizumab (targeting a different integrin) are in phase 3 trials. And by 2026, head-to-head trials like RHEA and VEGA will finally tell us which drug works best for which patient-based on biomarkers, not guesswork.
But the big question remains: can we prevent patients from cycling through multiple biologics? Right now, 30% of IBD patients switch classes within five years. That drives annual costs to $35,000-$75,000 per person. The goal isn’t just to treat-IBD care is moving toward stopping disease before it escalates.
Final Thoughts
IBD biologics aren’t just drugs. They’re lifelines. But they’re not simple. Each one has strengths, weaknesses, costs, and risks. The best choice isn’t the most powerful. It’s the one that works for you-your body, your lifestyle, your fears, and your finances. Talk to your doctor. Ask about biosimilars. Use patient support programs. And remember: if one doesn’t work, another might. The toolbox is bigger than ever. You just need to find the right tool.




13 Comments
Write a comment