Reperfusion Injury Treatment Optimizer
Choose Your Clinical Scenario
Optimal Treatment Strategy
Note: Timing is critical. Interventions must be administered before reperfusion for maximum effectiveness.
When a blocked artery is opened, the sudden rush of blood can paradoxically cause more damage. This phenomenon, known as reperfusion injury, is a double‑edged sword that clinicians wrestle with every day.
In medical terms, Reperfusion Injury is the tissue damage that occurs when blood supply returns to tissue after a period of ischemia. The lack of oxygen during the blocked phase (ischemia) primes cells for a cascade of events, and the re‑introduction of oxygen and nutrients can ignite a firestorm of harmful reactions.
Equally important is Inflammation is the body's complex biological response to harmful stimuli, involving immune cells, blood vessels, and molecular mediators. While inflammation is essential for healing, in the setting of reperfusion it often becomes excessive, amplifying tissue loss.
Why the Sudden Return of Blood Can Be Harmful
The core problem lies in the rapid shift from oxygen deprivation to oxygen abundance. This swing triggers three interlinked processes: oxidative stress, calcium overload, and an inflammatory surge.
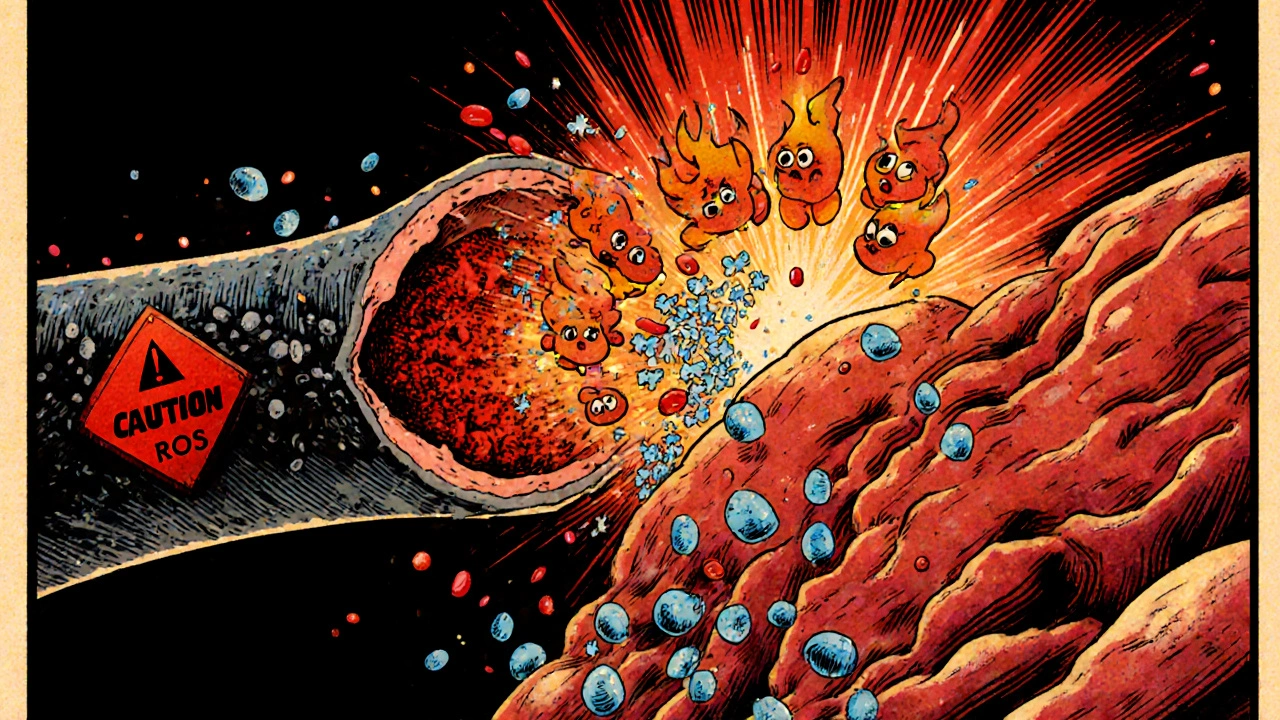
Oxidative Stress is the imbalance between the production of reactive oxygen species (ROS) and the body's ability to detoxify them. When blood flows back, mitochondria and enzymes like xanthine oxidase flood the tissue with ROS such as superoxide and hydrogen peroxide. These molecules attack lipids, proteins, and DNA, compromising cell integrity.
At the same time, the sudden influx of calcium ions overwhelms the cell's buffering systems-a phenomenon known as calcium overload. This overload activates proteases and phospholipases that further degrade cellular structures.
The inflammatory component is sparked by the damaged cells themselves. They release danger‑associated molecular patterns (DAMPs) that act as red flags for the immune system.
The Cellular Players That Turn Up the Heat
When DAMPs appear, the immune system sends in its first responders-Neutrophils are a type of white blood cell that rapidly migrates to sites of injury and releases enzymes and ROS. Within minutes, neutrophils adhere to the endothelium, roll along the vessel wall, and extravasate into the tissue.
Once inside, neutrophils unleash a barrage of enzymes (like elastase) and additional ROS, worsening oxidative stress. They also secrete pro‑inflammatory cytokines such as interleukin‑1β (IL‑1β) and tumor necrosis factor‑α (TNF‑α).
These cytokines activate the NF‑κB pathway is a key transcription factor cascade that regulates the expression of many inflammatory genes. NF‑κB moves into the nucleus and turns on genes for more cytokines, adhesion molecules, and chemokines, drawing even more immune cells into the area.
Another amplifier is the Complement System is a set of plasma proteins that, when activated, opsonize pathogens and damaged cells, and can form membrane‑attack complexes. Complement activation can happen directly on injured endothelium, leading to further vascular leakage and edema.
How the Blood Vessel Lining Gets Involved
The inner lining of blood vessels- the Endothelial Cells are cells that line the interior surface of blood vessels and regulate vascular tone, permeability, and leukocyte adhesion -is especially vulnerable. ROS and inflammatory cytokines cause endothelial dysfunction, reducing nitric oxide production and promoting vasoconstriction.
Endothelial cells also express adhesion molecules like ICAM‑1 and VCAM‑1, which act as docking stations for neutrophils and monocytes. This adhesion step locks immune cells in place, allowing them to release their destructive contents directly onto the vessel wall.
Clinical Scenarios Where the Link Matters
Heart attacks (myocardial infarctions) illustrate the problem vividly. After an occluded coronary artery is opened-whether by angioplasty, thrombolysis, or surgery-the heart muscle can suffer additional loss due to reperfusion injury. Studies estimate that up to 30% of final infarct size may be attributed to this process.
Stroke patients face a similar dilemma. Rapid restoration of cerebral blood flow is essential, yet the sudden surge of ROS and inflammation can enlarge the penumbra-the region of brain tissue that is still salvageable. Animal models show that anti‑inflammatory strategies can shrink the penumbra by 20%.
Organ transplantation also hinges on controlling reperfusion injury. When a donor organ is re‑vascularized in the recipient, the combined oxidative and inflammatory hit can dictate graft survival. Clinical protocols now include machine perfusion techniques that temper the initial burst of ROS.
Therapeutic Approaches: Turning Down the Volume
Because the injury cascade is multi‑layered, effective treatment usually combines several tactics.
- Antioxidants: Agents like N‑acetylcysteine (NAC) and vitamin C scavenge ROS. Clinical trials in cardiac patients report modest reductions in biomarkers of injury, though timing is critical-administration must occur before reperfusion.
- Anti‑inflammatory drugs: Corticosteroids, colchicine, and newer biologics targeting IL‑1β (e.g., canakinumab) have shown promise in reducing post‑myocardial infarction inflammation. The COLCOT trial demonstrated a 7% drop in major cardiovascular events with low‑dose colchicine.
- Ischemic Pre‑conditioning is a technique where brief, non‑lethal episodes of ischemia are applied before the main insult to build tolerance: Brief, non‑lethal episodes of ischemia before the main event “train” the heart to tolerate the oxidative surge. This strategy lowers ROS production and blunts NF‑κB activation.
- Therapeutic Hypothermia is controlled cooling of tissue to ~33°C that slows metabolism and reduces inflammatory signaling: Cooling the tissue to 33°C slows metabolic rates, reduces calcium influx, and limits inflammatory signaling. In cardiac arrest patients, hypothermia improves neurological outcomes.
- Mitochondrial Permeability Transition Pore is a protein channel that opens in mitochondria during reperfusion, leading to loss of membrane potential and cell death: Drugs like cyclosporine A block the pore that opens during reperfusion, preserving mitochondrial function and limiting cell death.
Importantly, timing is everything. Interventions given after the surge of ROS has already caused damage are far less effective. This reality has spurred research into rapid‑delivery systems, such as catheter‑based drug elution during angioplasty.
Comparing the Three Main Injury Mechanisms
| Mechanism | Primary Trigger | Major Cellular Effect | Typical Intervention |
|---|---|---|---|
| Oxidative Stress | Sudden oxygen influx | ROS damage to lipids, proteins, DNA | Antioxidants (NAC, vitamin C) |
| Calcium Overload | Disrupted ion pumps | Protease activation, mitochondrial dysfunction | Calcium channel blockers, hypothermia |
| Inflammatory Response | DAMP release & endothelial activation | Neutrophil infiltration, cytokine storm | Anti‑inflammatories, IL‑1β blockers |
Emerging Research Directions
Scientists are now exploring gene‑editing tools to knock out key inflammatory mediators in animal models. CRISPR‑based silencing of the NF‑κB subunit p65 reduced infarct size by 40% in mice.
Another hot area is nanotechnology. Lipid‑based nanoparticles loaded with both an antioxidant (e.g., superoxide dismutase mimetic) and an anti‑IL‑1β antibody have shown synergistic protection in rodent stroke models.
Finally, the gut microbiome is emerging as an indirect modulator. Certain bacterial metabolites can dampen systemic inflammation, potentially reducing the severity of reperfusion injury in distant organs.
Quick Checklist for Clinicians and Researchers
- Identify high‑risk patients (ST‑elevation MI, large‑vessel stroke) before reperfusion.
- Administer antioxidant therapy prior to revascularization when possible.
- Consider anti‑inflammatory agents (colchicine, IL‑1β blockers) as adjuncts.
- Use ischemic pre‑conditioning or controlled hypothermia where feasible.
- Monitor biomarkers (troponin, CRP, IL‑6) to gauge injury magnitude.
- Stay updated on trial data for mPTP inhibitors and nanoparticle delivery.
Frequently Asked Questions
What exactly triggers reperfusion injury?
The abrupt return of oxygenated blood after a period of ischemia generates reactive oxygen species, overwhelms calcium handling, and activates immune cells. Together these events damage cells that were already vulnerable.
Is inflammation always harmful after reperfusion?
Not always. A controlled inflammatory response helps clear debris and initiates repair. Problems arise when the response becomes excessive or prolonged, leading to extra tissue loss.
Can antioxidants completely prevent reperfusion injury?
Antioxidants reduce the oxidative burst but cannot block all pathways, such as calcium overload or immune cell activation. They are most effective when given before reperfusion and combined with other strategies.
What role does the complement system play?
Complement proteins can bind to damaged endothelial cells, forming membrane‑attack complexes that increase vascular leakage and attract more immune cells, amplifying inflammation.
Are there any approved drugs specifically for reperfusion injury?
There is no single FDA‑approved drug targeting reperfusion injury alone. However, agents like colchicine (for post‑MI inflammation) and cyclosporine (investigational mPTP inhibitor) are used off‑label or in clinical trials.
Putting It All Together
Reperfusion injury and inflammation are tightly linked, forming a feedback loop that can magnify tissue loss after a heart attack, stroke, or organ transplant. Understanding the underlying mechanisms-oxidative stress, calcium overload, neutrophil infiltration, cytokine storms, and endothelial dysfunction-helps clinicians choose the right combination of therapies at the right time. While no single magic bullet exists yet, a layered approach that includes antioxidants, anti‑inflammatory agents, pre‑conditioning, and emerging nanomedicines is the best bet for minimizing damage and improving outcomes.







We stand at the intersection of chemistry and fate, where the rush of blood after a blockage can feel like a national revival-bold, swift, and unapologetically American. Yet the same vigor that restores life also drags a hidden fire through our cells, a reminder that power without restraint is a reckless patriot. The cascade of ROS, calcium overload, and neutrophil frenzy reads like a cautionary tale for any empire that forgets humility. I could write a dissertation on it, but honestly, the literature already drags its feet long enough. In short, we must temper our heroic interventions with a sober respect for the body’s own limits.
Allow me to paint the scene with the vivid brushstrokes of a stage that refuses to dim: the arterial highway, once choked like a traffic jam on the Fourth of July parade, suddenly erupts in a deluge of crimson that splashes every corner of the myocardial theater. The oxygen, as if a spotlight suddenly blinding the actors, ignites a chorus of free radicals that pirouette across membranes, while calcium ions, those eager understudies, overflow onto the stage demanding their encore. Neutrophils, those overzealous extras, flood in with the enthusiasm of a new recruit at a rally, screaming cytokine slogans that echo through the vasculature. This drama, my friends, is not merely a medical mishap but a patriotic tragedy where our own zeal becomes the antagonist. In the grand narrative of healing, we must learn to cue the intermission before the climax turns into catastrophe.
Honestly, the hype around antioxidants feels like a meme that never aged-sure, they mop up some ROS, but they’re not a universal antidote, and the data is as mixed as a bipartisan debate. The timing of delivery is crucial; throw them in after the flood and you’re just sprinkling sugar on burnt toast. Moreover, the inflammatory cascade and calcium surge aren’t waiting for a vitamin C party to leave the stage. 📈🙂
Exactly, the antioxidant rush is like waving a 🇺🇸 flag at a fire-it looks proud, but the flame still burns. The real heroics come from coordinated strategies: pre‑conditioning, hypothermia, and a dash of targeted anti‑inflammatories. 🌟
Look, our system’s got the best tech on the planet, but we still throw a wrench in the works by rushing back blood like it’s a victory parade. If we want fewer hearts breaking after a heart attack, we need smarter protocols, not just louder drills.
One could argue the entire discourse on reperfusion is a hollow echo of hubris, where we mistake speed for wisdom. The cascade of damage is a philosophical reminder that every action carries a counter‑weight, and we neglect that balance at our peril. In truth, the rush to reopen vessels without pre‑emptive shields is a lesson in unchecked ambition.
The subtle dance between oxygen and inflammation is like a whispered secret that rarely gets shouted about. While the science talks about ROS and cytokines, we must remember the patient’s heartbeat, the silent pulse of hope that can be drowned by our own frantic interventions.
Permit me to intrude upon this quiet contemplation and pull back the curtain on the unnoticed players: the endothelial cells, those humble gatekeepers, suddenly find themselves besieged by a torrent of ROS and adhesion molecules, their once‑smooth surface now plastered with ICAM‑1 and VCAG‑1 like battle flags. This transformation isn’t just a footnote; it’s the very ground upon which neutrophils set up camp, releasing enzymes that gnaw at the vessel wall as if carving out a new path for chaos. Meanwhile, the complement system, a silent assassin in the background, drops membrane‑attack complexes like confetti, worsening edema and inviting more immune soldiers. All of these events cascade in a symphony of damage that could have been mitigated with a well‑timed antioxidant or a pre‑conditioning rehearsal. In short, the drama isn’t confined to the heart muscle; it spreads like wildfire across every vascular frontier, demanding our vigilant attention before we let the fire burn out of control.
It is both a privilege and a responsibility to synthesize the expansive body of knowledge surrounding reperfusion injury into actionable guidance for clinicians and researchers alike. First, we must acknowledge that the oxidative surge, calcium overload, and inflammatory cascade do not operate in isolation; they are interdependent threads woven into a single tapestry of cellular distress. By targeting these strands simultaneously-through the judicious use of antioxidants, controlled hypothermia, and anti‑inflammatory agents-we stand a better chance of preserving viable myocardium or cerebral tissue. Second, timing remains the linchpin of therapeutic success; pre‑emptive administration, such as delivering N‑acetylcysteine before balloon inflation, has repeatedly demonstrated superior outcomes compared with post‑reperfusion dosing. Third, emerging modalities, including nanoparticle‑mediated co‑delivery of superoxide dismutase mimetics and IL‑1β antibodies, offer the potential to synchronize antioxidant and anti‑inflammatory actions at the site of injury, thereby amplifying efficacy while minimizing systemic exposure. Fourth, the role of ischemic pre‑conditioning should not be relegated to historical curiosity; brief, controlled episodes of ischemia have been shown to up‑regulate endogenous protective pathways, including the activation of protein kinase C and the opening of mitochondrial ATP‑sensitive potassium channels, which collectively dampen ROS production. Fifth, therapeutic hypothermia, when applied promptly, reduces metabolic demand, limits calcium influx, and attenuates the nuclear translocation of NF‑κB, thus curbing the downstream cytokine storm. Sixth, ongoing clinical trials investigating mPTP inhibitors such as cyclosporine A highlight the necessity of preserving mitochondrial integrity during reperfusion, a strategy that may synergize with the aforementioned interventions. Seventh, clinicians should employ a multimodal monitoring approach, integrating biomarkers like troponin, CRP, and IL‑6 with imaging modalities to gauge the extent of injury and tailor therapy in real time. Eighth, the broader scientific community must continue to explore the gut microbiome’s modulatory capacity, as short‑chain fatty acids appear to temper systemic inflammation, potentially offering an adjunctive, non‑pharmacologic avenue for protection. Finally, education and protocol standardization across institutions are essential to ensure that these complex, layered strategies are implemented consistently and effectively. Moreover, interdisciplinary collaboration between cardiologists, neurologists, and transplant surgeons can accelerate the translation of these strategies into practice. Continuous feedback loops from clinical outcomes to laboratory research ensure that interventions remain adaptive to emerging challenges. Ultimately, the relentless pursuit of knowledge will outpace the vicious cycle of injury.
i think we need more real world data fast.
The journey from bench to bedside is a saga of perseverance, and every incremental discovery fuels the hope that one day we will tame the fiery aftermath of reperfusion. Let us rally together, celebrating each triumph, no matter how modest, for they are the lanterns guiding us through the darkness of uncertainty.
Some say the big pharma giants are quietly suppressing the most effective anti‑reperfusion formulas, keeping us in a cycle of endless trials and modest gains. It’s a classic playbook: the cure is out there, but the profit lies in the repeat procedures.
We have the tools it is simple we just need to use them more often!! The system should push for early antioxidants and cooling it will save lives!!
What a mess! they dnt want us to know the real truth about the hidden drugs that could stop the fire before it even starts!!! The whole medical establishment is a puppet show and the audience never sees the strings!!!