What Exactly Is a Corrective Action?
When a factory produces a batch of parts that don’t meet specs, what do they do? Stop the line? Throw out the bad pieces? That’s a correction. But if the same problem keeps happening week after week, you need something deeper. That’s where corrective action comes in.
Corrective action isn’t about fixing the symptom. It’s about finding out why the problem happened in the first place - and making sure it never happens again. Think of it like going to the doctor. If you have a headache, taking aspirin is a correction. Figuring out you’re dehydrated, drinking more water, and changing your routine? That’s corrective action.
In manufacturing, this process is often called CAPA - Corrective and Preventive Action. It’s not optional in regulated industries like medical devices, pharmaceuticals, or aerospace. The FDA, ISO, and other regulators require documented proof that manufacturers don’t just patch problems - they kill them at the source.
The Six Steps of a Real Corrective Action
There’s no magic formula, but every effective corrective action follows the same six steps. Skip one, and you’re just guessing.
- Identify the problem - It starts with data. A machine sensor spikes, a customer returns a part, an inspector catches a dimension out of tolerance. The key is catching it early through control points like statistical process control (SPC) charts or automated vision systems.
- Evaluate and categorize - Not all defects are equal. A cracked medical implant is a critical deviation. A slightly off-color label might be minor. Regulators like the FDA require risk-based categorization. Critical issues demand full CAPA. Minor ones can be handled with a simple correction log.
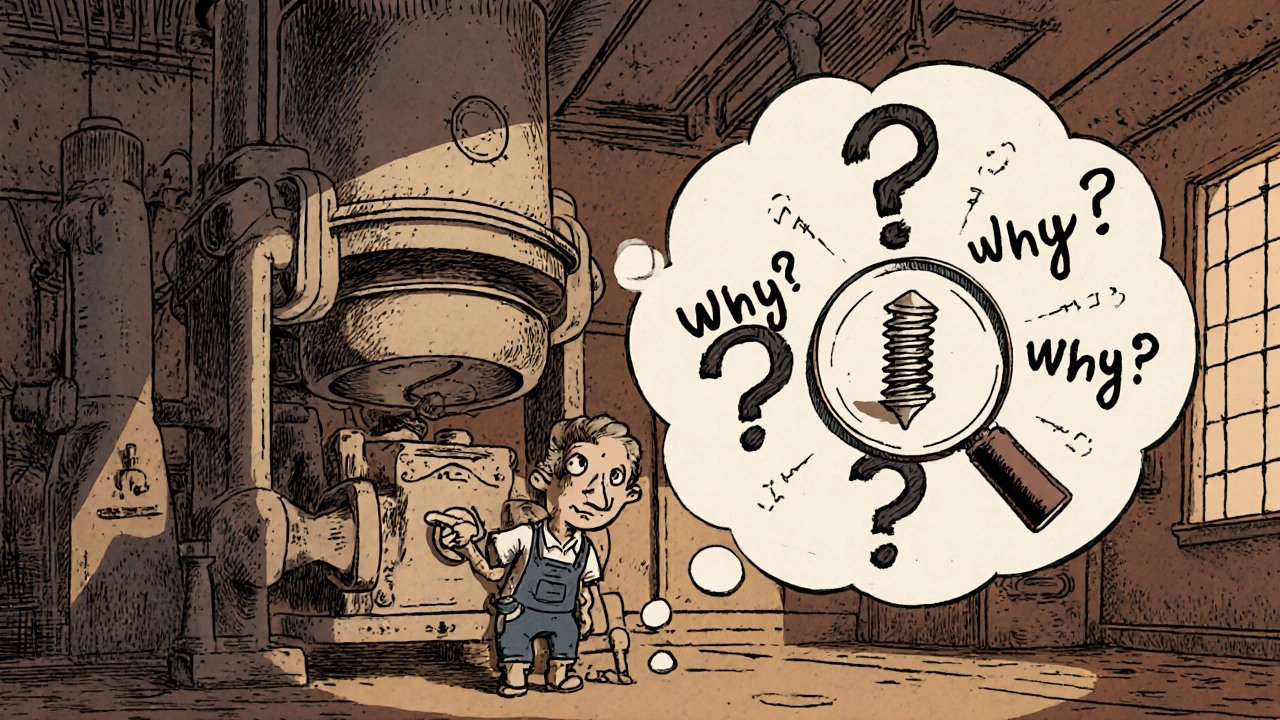
- Find the root cause - This is where most companies fail. Too many teams jump to conclusions: "The operator messed up." Or, "The machine is old." But the real cause? Maybe the operator wasn’t trained on the new jig. Maybe the machine’s calibration drifted because no one checked it in six months. Tools like the 5 Whys or Fishbone diagrams force you to dig deeper. One manufacturer found their weld failures weren’t due to operator skill - they were caused by humidity in the factory that no one had measured.
- Plan the fix - Once you know the cause, you design a solution. This isn’t vague. It must include: who will do it, by when, what exactly they’ll do, and how you’ll know it worked. Example: "Replace the calibration schedule for Machine #7 from monthly to weekly. Assign QA Lead Maria Chen to verify. Validate using 30 consecutive parts measured with calibrated CMM. Target: defect rate drop from 3.1% to under 0.5% within two production cycles."
- Implement the fix - Time to act. Update work instructions. Retrain staff. Modify tooling. Install new sensors. Document every change. This step fails when people say, "We told everyone," but no one actually updated the SOP or trained the night shift.
- Verify effectiveness - This is non-negotiable. Did the fix work? You don’t guess. You measure. You collect data. You compare defect rates before and after. You run statistical tests. If you’re in medical devices, you need to prove it with sample sizes of at least 30 units. If the defect rate didn’t drop by at least 50% after three production cycles, you go back to step three.
Why Most Corrective Actions Fail
Companies spend millions on quality systems. Yet, according to FDA warning letters from 2022, 61% of CAPA failures happen because teams fix symptoms, not causes.
Here are the three biggest reasons corrective actions collapse:
- Too much paperwork, not enough action - Some CAPA systems generate 47 pages of documentation for one issue. Quality teams spend more time filling out forms than fixing machines. The result? Delays. Frustration. Workarounds. The best systems use digital platforms that auto-populate fields, link data from machines, and reduce documentation time by 40% or more.
- No single owner - If everyone is responsible, no one is. A CAPA without a named person accountable for execution is a paperweight. The FDA explicitly requires assignment of responsibility. That person must have authority to get resources, stop production if needed, and follow through.
- No verification - "We fixed it," they say. But did they measure it? Did they watch for three weeks? Did they test 30 parts? Without hard data, you’re relying on hope. And hope doesn’t pass an audit.
One automotive supplier saw defect rates drop from 2.8% to 0.4% in 18 months - not because they bought new machines, but because they started assigning owners, cutting documentation by half, and requiring verification data before closing any CAPA.
Correction vs. Corrective Action vs. Preventive Action
People mix these up all the time. Here’s the difference:
| Type | Goal | When Used | Documentation Level |
|---|---|---|---|
| Correction | Fix the immediate issue | One-off mistake, minor deviation | Low - log entry, maybe a note |
| Corrective Action | Eliminate the root cause of a known problem | Problem repeats, affects safety or compliance | High - full CAPA with evidence |
| Preventive Action | Stop a problem before it happens | Pattern seen in data, risk identified | Medium - proactive plan based on trend analysis |
For example: If a screw keeps coming loose on a device (correction: tighten it manually). If it keeps happening because the torque setting on the screwdriver drifted (corrective action: recalibrate tools and add daily checks). If you notice similar screws on other models are starting to show wear patterns (preventive action: update torque specs across all product lines).
According to Cognidox, 68% of quality failures happen because teams treat corrections like corrective actions - and miss the real problem.
What Regulators Really Want
Regulators aren’t trying to punish manufacturers. They want patient safety. They want reliable products. And they know that without real corrective actions, problems come back.
The FDA’s 2023 guidance says it plainly: "Corrective actions must be verified and validated." In 2022, 28% of all quality system observations in medical device inspections were about CAPA failures - second only to document control issues.
What does "verified and validated" mean? It means:
- You didn’t just say you fixed it - you showed data proving it.
- You didn’t just update a document - you trained people and checked their work.
- You didn’t just fix one batch - you proved the fix works across multiple production runs.
Dr. John Snow from the FDA’s Office of Compliance said in a 2022 webinar: "The most common CAPA failure is addressing symptoms rather than root causes."
ISO 13485:2016 requires manufacturers to prove corrective actions "eliminate the causes of nonconformities." That’s not a suggestion. It’s a legal requirement.
Companies with strong CAPA systems see 27% fewer field actions and 34% less regulatory scrutiny, according to MedTech Consulting’s analysis of 157 firms.
How Technology Is Changing Corrective Actions
Old-school CAPA meant paper forms, filing cabinets, and delayed responses. Today, the best manufacturers are using digital systems that connect directly to machines.
Here’s what’s working now:
- AI-powered root cause analysis - Systems that analyze sensor data from 200 machines and suggest the top 3 likely causes of a defect. One manufacturer cut investigation time by 52%.
- Real-time dashboards - Quality managers see defects pop up on a screen the moment they happen. No waiting for daily reports.
- Automated CAPA triggers - If a machine’s defect rate crosses a threshold, the system auto-creates a CAPA ticket, assigns it, and sets deadlines.
- Blockchain audit trails - For regulated industries, some are testing tamper-proof digital logs that prove every step of the CAPA was done, when, and by whom.
Gartner predicts 65% of manufacturers will use predictive CAPA systems by 2027 - systems that flag risks before defects even occur. That could cut quality-related downtime by nearly half.

When Is CAPA Overkill?
Not every factory needs a full FDA-style CAPA system.
If you make custom metal brackets in small batches with 50 units per order and have a defect rate of 0.2%, you probably don’t need 47-page CAPAs for every hiccup. A simple log with a quick root cause and fix is enough.
The key is proportionality. Regulators expect you to match your system to your risk. Medical device makers? Full CAPA. A small bakery making custom cake toppers? A simple internal review form.
Don’t let bureaucracy kill your efficiency. But don’t ignore real patterns either. If the same problem shows up twice, it’s time to dig deeper.
What Success Looks Like
Successful corrective actions don’t just fix problems - they change culture.
At one medical device company, engineers used to hide defects to avoid blame. After implementing a no-blame CAPA process focused on learning, not punishment, defect reports increased by 40% in the first month. Why? Because people finally felt safe reporting issues. Within a year, overall defect rates dropped 62%.
Another company reduced quality-related downtime by 37% and cut operational costs by 19% - not by buying new machines, but by fixing the same problems over and over again, the right way.
The data is clear: when manufacturers stop treating symptoms and start killing root causes, they save money, satisfy customers, and pass audits with ease.
Getting Started Today
You don’t need a $500,000 software system to start fixing quality problems properly.
Here’s your 3-step starter plan:
- Pick one recurring problem - Find the defect that shows up every week. Don’t try to fix everything.
- Use the 5 Whys - Ask "why?" five times. Write down each answer. Don’t stop until you hit something you can control - like a missing step in training, a broken tool, or a misaligned fixture.
- Fix it, then measure it - Do one thing to fix the root cause. Track the defect rate for the next three production runs. If it doesn’t drop by at least half, you didn’t find the real cause.
That’s it. No forms. No software. Just focus, dig deep, and prove it worked.
Corrective action isn’t about perfection. It’s about progress. And progress beats excuses every time.
What’s the difference between a correction and a corrective action?
A correction fixes the immediate problem - like throwing out a bad part or adjusting a machine setting. A corrective action finds and eliminates the root cause so the problem doesn’t happen again. Corrections are quick fixes. Corrective actions are permanent solutions.
Do I need a CAPA system if I’m not in the medical industry?
You don’t legally need a formal CAPA system unless you’re regulated - like in medical devices, pharmaceuticals, or aerospace. But even small manufacturers benefit. If you have recurring quality issues, a simple version of CAPA helps you stop wasting time and money fixing the same problem over and over. It’s about efficiency, not just compliance.
Why do so many corrective actions fail?
Most fail because teams stop at the symptom. They blame the operator or the machine without digging deeper. They don’t assign clear ownership. And worst of all, they don’t verify the fix actually worked. Without data showing reduced defects over time, it’s just paperwork.
How long should a corrective action take?
There’s no fixed timeline, but it should be proportional to the risk. A minor issue might be resolved in a week. A major problem affecting safety could take months. The key is not speed - it’s thoroughness. Rushing leads to recurrence. The FDA warns that 61% of CAPA failures happen because root causes weren’t properly identified.
Can software really improve corrective actions?
Yes - but only if it connects to real data. Software that auto-creates CAPAs from machine alerts, tracks verification results, and reduces paperwork by 40% is powerful. But software that just replaces paper forms with digital ones adds no value. The best tools link quality data to production data and make root cause analysis faster and more accurate.
What’s the biggest mistake manufacturers make with CAPA?
The biggest mistake is treating CAPA as a compliance chore instead of a problem-solving tool. When teams see it as paperwork to get through, they skip steps, rush investigations, and skip verification. The best companies treat CAPA as their most important quality improvement program - and measure success by how many problems they stopped before they happened.







19 Comments
Write a comment