When you pick up a prescription for a generic medication, you might notice something odd: the same drug comes in different pills, with different names on the label. One bottle says Apotex, another says Teva, and a third says Actavis. They all contain the same active ingredient - say, lisinopril - and they all cost less than the brand-name version. But are they all the same? And if not, how do you pick the right one?
Not All Generics Are Created Equal
It’s easy to assume that if two drugs have the same name and dose, they work the same way. That’s mostly true - but not always. The U.S. Food and Drug Administration (FDA) requires all generic drugs to prove they deliver the same amount of active ingredient into your bloodstream as the brand-name version. This is called bioequivalence. The rule? The generic’s absorption rate must fall within 80% to 125% of the brand’s. That sounds wide, but in practice, most generics are within 5% of the original. Still, that small range means some generics can behave slightly differently in your body. This isn’t about quality or safety. All FDA-approved generics are safe. But for certain drugs, even tiny differences matter. Think of it like two identical cars with slightly different tires. Both will get you down the highway. But if you’re racing, or driving on icy roads, the tire choice makes a difference.What Makes a Generic Riskier?
Some medications have what’s called a narrow therapeutic index. That means the difference between a dose that works and a dose that’s dangerous is very small. For these drugs, switching between generics - or from brand to generic - can cause problems. Drugs in this high-risk group include:- Warfarin (blood thinner)
- Levothyroxine (for hypothyroidism)
- Digoxin (for heart rhythm)
- Phenytoin (for seizures)
- Cyclosporine (for organ transplants)
The Orange Book: Your Secret Weapon
The FDA publishes a public database called the Orange Book. It lists every approved drug - brand and generic - and gives each one a therapeutic equivalence (TE) code. This code tells you if a generic is a direct substitute. Here’s what the codes mean:- AB: This generic has been tested and proven to work the same as the brand. It’s fully interchangeable. Most generics you’ll see fall here.
- B: This generic meets FDA standards, but there’s some uncertainty about whether it behaves exactly like the brand. These are often newer generics or complex formulations. Use with caution, especially if you’re already stable on a brand or another generic.
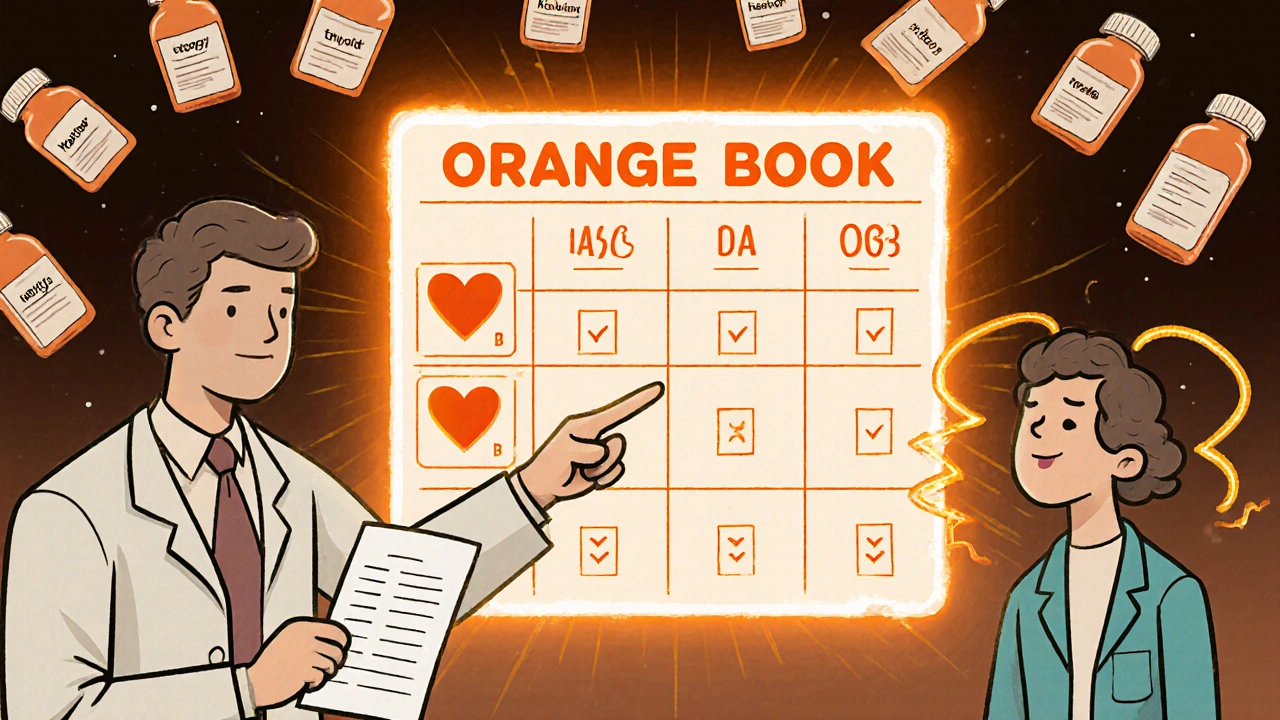
When Should You Stick With One Generic?
If you’ve been taking a generic for months and your condition is stable - your blood pressure is controlled, your thyroid levels are normal, your seizures are gone - don’t switch unless you have to. Even if the new generic is cheaper or has a different name, consistency matters. A 2023 review in UCSF Magazine found that patients who switched between multiple generic manufacturers of levothyroxine were more likely to need dose adjustments. Why? Because different manufacturers use slightly different inactive ingredients (fillers, coatings, binders). These don’t affect the active drug, but they can change how quickly it dissolves in your gut. For most people, that’s fine. For someone with thyroid disease, even a 10% change in absorption can throw off their entire treatment. So if you’re doing well on a Teva version of levothyroxine, stay on it. Don’t let the pharmacy switch you to an Apotex version just because it’s 50 cents cheaper. Ask them to keep you on the same brand.What If You’re Starting Fresh?
If you’ve never taken the drug before - say, you’ve just been diagnosed with high blood pressure - you have more flexibility. In that case, go with the cheapest AB-rated generic. There’s no reason to pay more for a brand when a generic works just as well. But here’s a pro tip: even when you start with a generic, ask your pharmacist to note which manufacturer it is. Write it down. Or keep the bottle. That way, if you ever need to refill and the pharmacy tries to switch you to a different one, you’ll know.Pharmacists Are Your Allies
In most states, pharmacists can switch your brand-name prescription to a generic unless your doctor writes “dispense as written.” But in 28 states, they’re required to tell you - or your doctor - if they’re switching to a different manufacturer than what you used last time. That’s not a flaw. It’s a safeguard. Pharmacists are trained to check the Orange Book and know which generics are safest for your condition. If you’re on warfarin and the pharmacy tries to switch you from one generic to another, they should flag it. If they don’t, ask. Don’t assume the cheapest option is always the best. Ask: “Is this the same manufacturer as last time?” If not, ask if it’s AB-rated. If you’re unsure, call your doctor. It’s better to be safe than sorry.
Why Do So Many Generics Exist?
There’s a simple reason: competition. As of October 2023, the FDA listed over 14,000 generic versions of just 2,745 active ingredients. That’s an average of five manufacturers per drug. When more companies make the same drug, prices drop. In 2022, generics saved the U.S. healthcare system $373 billion. But that same competition creates confusion. You might get one generic this month, another next month. That’s fine for most drugs. But for those with narrow therapeutic indices, it’s risky. The FDA is responding. In 2021, they issued warnings to levothyroxine manufacturers over inconsistent bioequivalence. New testing rules went into effect in 2022. And now, Congress is considering a bill that would require each generic manufacturer to have a unique barcode (NDC code) so doctors and pharmacists can track exactly which version you’re taking.What You Can Do Right Now
Here’s a simple checklist to help you make smart choices:- Check your prescription. Is it for a brand-name drug or a generic? If it’s brand, ask if a generic is okay.
- Ask your pharmacist. “Is this AB-rated?” If it’s not, ask why.
- Know your drug. Is it for thyroid, heart, epilepsy, or blood thinning? If yes, stick with one manufacturer.
- Keep the bottle. Write down the manufacturer name. Use it as a reference for future refills.
- Don’t assume cheaper is better. A 10-cent difference isn’t worth a trip to the ER.
- Speak up. If you feel different after switching - tired, dizzy, heart racing - tell your doctor and pharmacist. It might be the generic.
Final Thought: Generics Are Good - But Consistency Is Better
Generics aren’t inferior. They’re essential. They make life-saving drugs affordable. But for certain conditions, your body doesn’t care about price tags - it cares about consistency. If you’re stable on a generic, stay on it. If you’re starting out, pick the cheapest AB-rated one. And always, always ask questions. Your health isn’t a commodity. It’s your life.Are generic medications as safe as brand-name drugs?
Yes. All FDA-approved generics must meet the same strict safety and quality standards as brand-name drugs. They contain the same active ingredient, in the same strength, and are made in the same type of facilities. The only differences are in inactive ingredients like fillers or coatings, which don’t affect how the drug works - but can matter for some people.
Can I switch between different generic brands of the same drug?
For most medications, yes - as long as they’re AB-rated. But for drugs with a narrow therapeutic index - like warfarin, levothyroxine, or digoxin - switching between manufacturers can cause changes in blood levels that lead to side effects. If you’re stable on one generic, stick with it. Don’t switch unless your doctor recommends it.
What does AB-rated mean on a generic drug?
AB-rated means the generic has been proven by the FDA to be bioequivalent to the brand-name drug. It delivers the same amount of active ingredient into your bloodstream at the same rate. These are considered fully interchangeable. You can safely switch between different AB-rated generics - but again, for sensitive drugs, staying with one manufacturer is best.
Why do some generics cost more than others?
Price differences come down to manufacturing costs, market competition, and supply chain factors. Sometimes, a newer generic manufacturer charges more to recoup development costs. Other times, an older manufacturer has lower overhead. It doesn’t mean one is better - just that pricing isn’t always tied to quality. Always check the therapeutic equivalence rating, not the price tag.
Should I ask my doctor to write “dispense as written” on my prescription?
Only if you’re taking a drug with a narrow therapeutic index and you’ve found a specific generic that works well for you. Otherwise, it’s usually unnecessary. Most generics are safe and effective. Writing “dispense as written” can increase your out-of-pocket cost and limit your access to affordable options. Talk to your pharmacist first - they can help you decide if staying on one brand is worth the extra cost.
How do I know which manufacturer made my generic drug?
Look at the pill bottle or box. The manufacturer’s name is usually printed near the bottom or on the side. You can also ask your pharmacist for the name of the company that made your medication. Write it down or take a photo. That way, if you refill and the pill looks different, you’ll know it’s a new manufacturer - and you can ask if it’s the same one you’ve been taking.







I’ve been on levothyroxine for six years and switched generics twice-both times I felt like a zombie for two weeks. Never again. I now keep the bottle and show it to the pharmacist. They roll their eyes but they do it.
Consistency isn’t fancy, but it’s life-saving.
Actually, the FDA’s 80–125% bioequivalence window is dangerously wide for drugs like warfarin. Many countries use 90–110%. The U.S. system is outdated and prioritizes cost over precision. I’ve seen patients hospitalized because pharmacies switched generics without warning. This isn’t just about pills-it’s about regulatory failure.
Let’s be real: the entire generic drug system is a capitalist illusion. We’re told ‘it’s the same drug’-but the inactive ingredients? The manufacturing environment? The batch-to-batch variability? These aren’t trivial. They’re the silent variables in a biological equation we pretend we’ve solved. And now we wonder why people feel ‘off’ after a switch…
The Orange Book? It’s a spreadsheet, not a guarantee. The FDA doesn’t test every batch. They test one. One. And then we all get to be lab rats.
It’s not about trust. It’s about systemic negligence dressed up as efficiency.
Generics are one of the few beautiful things about American medicine-they make survival affordable. But here’s the truth: our bodies aren’t machines. They’re delicate ecosystems. A 5% difference in absorption? For someone with a thyroid condition, that’s like changing the rhythm of their heartbeat.
It’s not about being anti-generic. It’s about honoring the individual. If your body’s found peace with Teva, let it rest. Don’t let a 30-cent savings cost you sleep, energy, or peace of mind.
Medicine isn’t a commodity. It’s a conversation between your biology and the science that listens. Treat it that way.
So let me get this straight-you’re telling me I should pay more to avoid a 50-cent savings because some pharmacist might swap my pills?
Bro. I’ve taken 12 different generics of lisinopril. I’m fine. The system works.
Stop overthinking it.
Here’s the data: 2017 Circulation study showed a 22% spike in adverse events after switching candesartan generics. But here’s the kicker-most patients don’t report it. Why? Because they assume it’s ‘just aging’ or ‘stress.’ The real problem isn’t the generics-it’s the lack of pharmacovigilance. We track drug sales, not patient outcomes. That’s not oversight. That’s negligence.
Y’all are making a big deal outta nothing. FDA says its good so its good. Why you think they approve it if its dangerous? You think Big Pharma is hiding somethin? Nah. They want you to buy brand name. Generics are cheaper because they dont pay for ads. Simple. Stop being paranoid. I take 3 generics and feel fine.
Biggest tip I ever got: write the manufacturer on your calendar next to your meds. I do it for my mom’s digoxin. She’s 78, and last year they switched her from Mylan to Sandoz-she got dizzy for a week. We caught it because we had a note. Pharmacist apologized. Now she’s back on Mylan. Simple. Free. Life-changing.
👍
Just stick with one. I learned the hard way. Switched my blood pressure med, felt like crap for a month. Turned out it was the filler. Now I only take the one with the blue dot. Don’t overthink it. Just keep it the same.
It is imperative to note that the FDA’s therapeutic equivalence codes are not merely recommendations-they are legally binding classifications under the Drug Price Competition and Patent Term Restoration Act of 1984. Therefore, any deviation from AB-rated generics without clinical justification constitutes a breach of established pharmaceutical standards, and pharmacists are obligated to adhere to this protocol.
Let’s cut the crap. The only reason generics exist is to let big pharma profit off patents while outsourcing production to the cheapest factory in India or China. The FDA doesn’t inspect those plants. They get reports. Reports. That’s it. You think your ‘safe’ generic isn’t made in a warehouse with no AC and rats running through the powder? Wake up.
I used to switch ‘cause it was cheaper. Then I got dizzy. Now I just ask ‘same as last time?’ If not, I say no. Easy. My doctor doesn’t care. But I do.
Simple.
In South Africa, we don’t have the luxury of choice. We take what’s available. But here’s what I’ve learned: if your medication makes you feel like yourself, don’t touch it. If you start feeling off, it’s not ‘just stress’-it’s the pill. Talk to your pharmacist. Ask for the name. Write it down. We don’t have the Orange Book, but we have our bodies. Trust them.
Stop treating your meds like a lottery ticket. If you’re on something that keeps you alive-thyroid, heart, seizure meds-don’t gamble with the brand. I used to think ‘it’s all the same’ until my cousin went into atrial fibrillation after a switch. Now she only takes the one with the red stripe. And she’s alive. That’s not luck. That’s smart.
Don’t be cheap with your health. You’re worth more than 50 cents.
Ever wonder why the same generic looks different every time? It’s not random. The FDA lets them change the coating to avoid lawsuits. They know if you get sick, you’ll blame the drug-not the manufacturer. They’re playing you. The barcode system? It’s coming. But only because people are starting to notice. You think this is coincidence? Nah. It’s control.