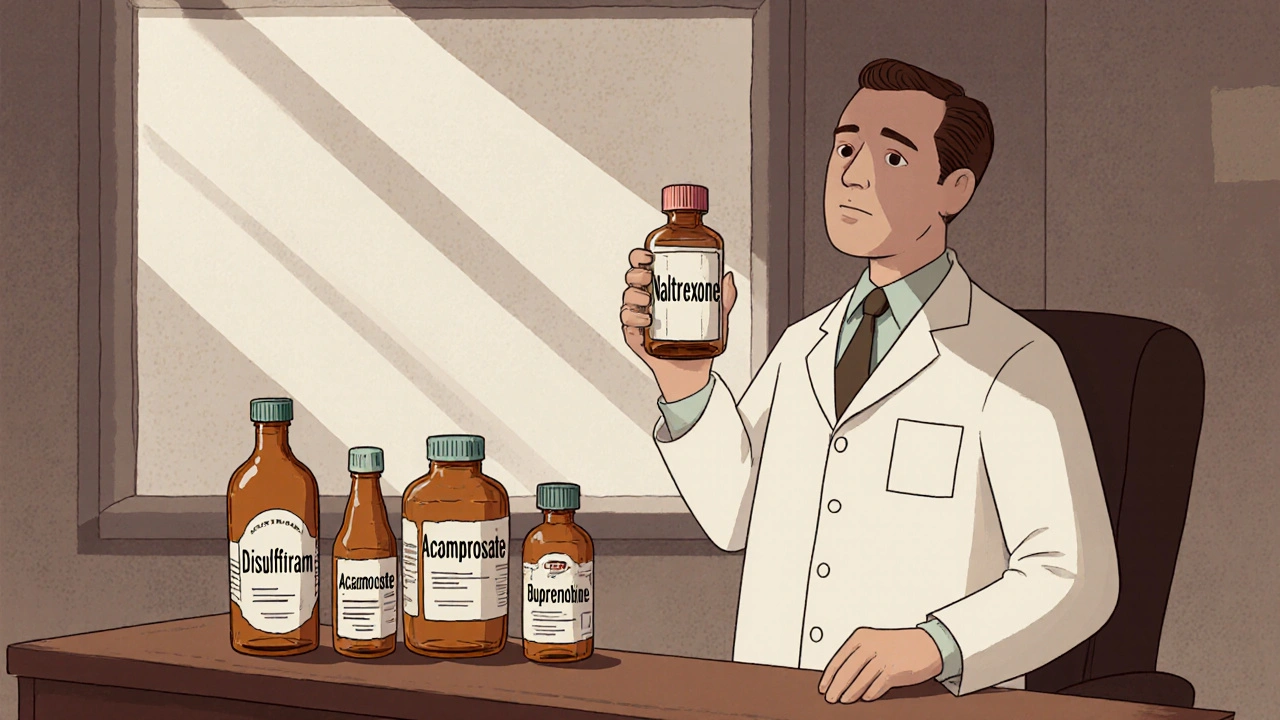
When doctors need to curb cravings for alcohol or opioids, they often reach for an opioid antagonist called Naltrexone. It works by blocking the brain’s reward receptors, making the substance feel less rewarding. But Naltrexone isn’t the only option on the shelf, and each alternative brings its own set of strengths and trade‑offs. This guide walks you through the most common substitutes, explains how they differ, and helps you decide which one fits a particular clinical picture.
Why compare Naltrexone with other medications?
Choosing the right drug isn’t a one‑size‑fits‑all decision. Patients vary in liver health, concomitant medicines, the type of dependence (alcohol vs. opioids), and how quickly they need relief. By laying out the mechanisms, dosing regimens, and side‑effect profiles side‑by‑side, you can match a medication to a patient’s needs without guessing.
Core characteristics of each option
The table below captures the most relevant data points for five widely used agents: Naltrexone, Disulfiram, Acamprosate, Naloxone, and Buprenorphine. All figures are based on the latest UK prescribing guidelines (2024) and FDA label information.
| Drug | Mechanism | Primary Indication | Typical Dose Form | Onset of Action | Common Side Effects |
|---|---|---|---|---|---|
| Naltrexone | Opioid‑receptor antagonist (μ‑blockade) | Alcohol Use Disorder, Opioid Use Disorder | Oral 50 mg tablet; Injectable 380 mg monthly (Vivitrol) | 30 min (oral), 24‑48 h (injectable) | Nausea, headache, fatigue |
| Disulfiram | Inhibits aldehyde dehydrogenase → acetaldehyde buildup | Alcohol Use Disorder (deterrent) | Oral 250‑500 mg tablet | 6‑12 h | Metallic taste, flushing, hypotension |
| Acamprosate | Modulates glutamate & GABA neurotransmission | Alcohol Use Disorder (maintenance) | Oral 666 mg tablet (2 tablets bid) | 12‑24 h | Diarrhea, abdominal pain, insomnia |
| Naloxone | Rapid opioid‑receptor antagonist (μ‑blockade) | Acute opioid overdose reversal | Injectable 0.4 mg/mL, nasal spray 4 mg | 1‑2 min (IV), 5‑10 min (nasal) | Withdrawal symptoms, tachycardia |
| Buprenorphine | Partial μ‑agonist, κ‑antagonist | Opioid Use Disorder (maintenance) | Sub‑lingual 2‑8 mg tablet, trans‑dermal patch | 30‑60 min | Constipation, dizziness, mild euphoria |
Deep dive into each alternative
Disulfiram works like a chemical roadblock: when alcohol is consumed, acetaldehyde can’t be broken down, causing an intense flush and nausea. It’s great for patients who are highly motivated to stay sober because the unpleasant reaction is a strong deterrent. However, the drug demands strict abstinence-any accidental sip of alcohol (including cough syrup) can be dangerous. Liver enzymes need monitoring because the medication itself is hepatotoxic.
Acamprosate is a “maintainer” rather than a blocker. It helps restore the balance between excitatory (glutamate) and inhibitory (GABA) pathways that become disrupted during chronic drinking. The biggest advantage is its safety profile; it’s renally excreted, so it doesn’t stress the liver. The downside is that it must be taken three times daily, which can hurt adherence.
Naloxone isn’t a treatment for dependence; it’s an emergency rescue drug. When administered during an overdose, it rapidly displaces opioids from receptors, reversing respiratory depression. Because it can precipitate severe withdrawal, it’s only used in acute settings, not for long‑term relapse prevention.
Buprenorphine occupies opioid receptors partially, delivering enough activation to curb cravings while limiting the “high”. Its ceiling effect reduces overdose risk, making it a popular choice for office‑based treatment. Some patients experience mild euphoria, and its partial agonism can still cause physical dependence, so a gradual taper is needed after stabilization.
When Naltrexone shines
Because Naltrexone blocks receptors rather than creating a new pharmacologic stimulus, it doesn’t produce dependence. This makes it a good fit for people who want a medication‑free lifestyle after the initial stabilization phase. The oral formulation is cheap and easy to start, while the injectable brand (Vivitrol) offers once‑monthly dosing, which improves adherence for patients struggling with daily pills.
Two clinical pearls help you get the most out of Naltrexone:
- Ensure the patient is opioid‑free for at least 7‑10 days before the first dose to avoid precipitated withdrawal.
- Check liver enzymes (ALT, AST) at baseline and periodically, especially if the patient has hepatitis C or heavy alcohol use.
Choosing the right drug - a decision matrix
Below is a quick‑reference matrix that matches patient characteristics to the most suitable medication.
- Patient wants minimal daily pills: Injectable Naltrexone (Vivitrol) or Buprenorphine patch.
- Liver disease present: Acamprosate (renal clearance) or Buprenorphine (minimal hepatic metabolism).
- High motivation, no relapse risk: Disulfiram as a deterrent.
- Recent opioid use, high overdose risk: Buprenorphine for maintenance, keep Naloxone on hand for emergencies.
- Needs rapid reversal of opioid overdose: Naloxone, administered by EMS or bystanders.
Potential pitfalls and how to avoid them
Even the best‑studied drugs can backfire if prescribers ignore key contraindications.
- Pre‑existing opioid use - Starting Naltrexone too soon can trigger severe withdrawal. Verify abstinence with a urine screen.
- Alcohol-disulfiram interaction - Patients often forget to avoid hidden sources of alcohol (e.g., certain mouthwashes). Provide a comprehensive list of alcohol‑containing products.
- Renal insufficiency - Acamprosate accumulates in kidney failure; dose‑adjust or avoid.
- Pregnancy - Most of these agents lack robust safety data; discuss risks versus benefits openly.
Key takeaways
- Naltrexone blocks opioid receptors without causing dependence; ideal for motivated patients.
- Disulfiram is a deterrent that requires strict abstinence.
- Acamprosate offers a safe maintenance option for liver‑compromised patients.
- Naloxone is an emergency reversal drug, not a long‑term therapy.
- Buprenorphine provides partial agonist benefits with a lower overdose ceiling.
Frequently Asked Questions
Can I take Naltrexone and Disulfiram together?
Combining the two isn’t recommended. Disulfiram relies on an intact alcohol‑metabolism pathway, while Naltrexone blocks opioid receptors. Using both can increase side‑effect burden without added benefit.
How long does the injectable Naltrexone last?
Vivitrol is administered as a 380 mg intramuscular injection once every 28 days. Blood levels stay therapeutic for the full month, which helps patients who miss daily oral doses.
Is Acamprosate safe for people with kidney disease?
Acamprosate is primarily cleared by the kidneys. In moderate to severe renal impairment (creatinine clearance <30 mL/min) the drug should be avoided or dose‑reduced under specialist supervision.
When should Naloxone be given during an overdose?
If the person is unresponsive, has shallow breathing, or shows pinpoint pupils, administer 0.4 mg IV or 4 mg nasally immediately. Repeat every 2‑3 minutes until respiration improves.
Can Buprenorphine be used for alcohol dependence?
Buprenorphine is not indicated for alcohol use disorder. While it can reduce cravings for opioids, it does not address the neurochemical pathways driving alcohol dependence.
By matching the drug’s pharmacology to the patient’s clinical picture, you can maximize success rates and minimize unwanted effects. Whether you pick naltrexone, Disulfiram, Acamprosate, or a rescue agent like Naloxone, the key is a clear treatment plan, regular monitoring, and honest conversations about goals.






Oh wow, I totally forget how scared I was before starting naltrexone, but it turned out to be a real lifesaver cuz it helped me stay clean! :)
When you compare naltrexone to its alternatives, a few key pharmacologic distinctions emerge; first, naltrexone acts as a pure μ‑opioid antagonist, which means it blocks the receptor without providing any agonist activity, thereby avoiding new dependence, unlike buprenorphine which is a partial agonist.
Second, the onset of action for oral naltrexone is roughly 30 minutes, whereas disulfiram requires several hours to manifest its aversive response after alcohol ingestion.
Third, the side‑effect profile of naltrexone-primarily nausea, headache, and fatigue-is generally milder than the metallic taste and flushing seen with disulfiram.
Fourth, the hepatic considerations are notable; baseline liver enzymes should be checked before initiating naltrexone, especially in patients with heavy alcohol use or hepatitis C, whereas acamprosate is cleared renally and spares the liver.
Fifth, adherence can be dramatically improved with the injectable formulation (Vivitrol), which provides steady plasma levels for a full 28‑day period, mitigating the daily‑pill burden that some patients find challenging.
Sixth, the contraindication of recent opioid use is crucial: initiating naltrexone too early can precipitate severe withdrawal, so a 7‑10 day opioid‑free window confirmed by urine toxicology is standard practice.
Seventh, disulfiram’s efficacy hinges on patient motivation; it is essentially a deterrent that only works if the patient is committed to complete abstinence, because any accidental alcohol exposure can cause dangerous reactions.
Eighth, acamprosate’s mechanism of modulating glutamate and GABA balances neurochemical homeostasis, making it useful for maintaining abstinence after detox, yet its thrice‑daily dosing schedule can impede compliance.
Ninth, naloxone, while lifesaving in overdose scenarios, is not a maintenance therapy; its rapid onset (1‑2 minutes IV) and short duration make it unsuitable for craving control.
Tenth, buprenorphine’s ceiling effect reduces overdose risk, yet it still carries a risk of physical dependence and may require a gradual taper after stabilization.
Eleventh, patient-specific factors such as liver disease, renal insufficiency, pregnancy status, and co‑occurring psychiatric conditions should guide drug selection, with acamprosate preferred for hepatic compromise and buprenorphine or naltrexone considered when renal function is intact.
Twelfth, cost considerations matter: oral naltrexone is inexpensive, while the injectable form and buprenorphine patches can be pricier, potentially limiting access for uninsured patients.
Thirteenth, insurance formularies often favor certain agents, so clinicians need to be familiar with prior‑authorisation requirements for Vivitrol and buprenorphine.
Fourteenth, patient education about hidden sources of alcohol (e.g., mouthwashes) is essential when prescribing disulfiram, to prevent inadvertent toxicity.
Fifteenth, regular follow‑up visits to monitor liver enzymes, renal function, and adherence patterns are vital regardless of the chosen medication.
Sixteenth, ultimately, the decision matrix should balance efficacy, safety, patient preference, and practical considerations to optimize outcomes.
This guide does a solid job of laying out the pharmacology, but I’d add a global lens: in many European countries, the injectable form of naltrexone is more commonly used for alcohol dependence because of its adherence benefits, while in parts of Asia, acamprosate is often preferred due to concerns about liver health.
Yo! Great rundown – I’ve seen patients bounce between disulfiram and naltrexone, and the key is keeping them motivated; remember to give them a pep talk before they start, cuz a little hype can go a long way! 😎
For anyone weighing the options, think of it as a toolbox: if the patient wants a low‑maintenance route, Vivitrol is like the power drill that just clicks in and works; if they have liver concerns, acamprosate is the safe screwdriver that won’t damage the wood. Tailor the choice to their lifestyle and health status, and follow up regularly to tweak the plan.
Love how concise this is! 🌟 It really helps demystify the options for folks just starting their recovery journey.
From a clinical governance perspective, the matrix presented aligns well with current NICE guidelines: prioritize naltrexone for patients without hepatic impairment, consider buprenorphine for opioid‑use disorder with a safety ceiling, and reserve disulfiram for highly motivated individuals who can adhere to strict abstinence.
Good to see the emphasis on urine screens before naltrexone; in my practice, I also incorporate a brief motivational interview to ensure the 7‑10 day opioid‑free window is realistic for the patient.
Philosophically, each medication reflects a different stance on addiction: naltrexone says “let’s block the reward,” while buprenorphine says “let’s replace it with something gentler.” Both paths aim for autonomy, but the journey feels distinct. 🧠
Indeed, the contrast you draw is insightful. While the blocker approach reduces cravings without creating new dependence, the partial agonist offers a smoother transition for those fearing withdrawal. Both philosophies can coexist in a patient‑centered plan.
Your detailed breakdown of onset times and side‑effect profiles is very helpful; clinicians will appreciate the clear comparison when tailoring treatment strategies.